Gold heap leaching with an alkaline solution as sodium cyanide leaching solution, leaching the ore is not strong multi-acidic rocks. If the acidity of the rock is strong, the washing pretreatment is carried out until the pH of the washing water flowing out of the washing is ≥9, so that sodium cyanide leaching can be performed. Therefore, its fouling situation is divided into two stages to discuss, one is the heap washing stage, and the other is the leaching stage. If lime granulation heap leaching and pre-cyanide (sodium cyanide solution are added during granulation), the heap washing is no longer carried out.
The acidity of the ore, the scaling in the heap washing stage is very noteworthy, although the washing water of the heap does not enter the aftertreatment system, it will not cause the equipment and pipeline fouling of the carbon adsorption system, but if it is not handled properly, it will The surface of the ore heap, the inside of the heap and the sprinkling system are severely fouled. The wear of the nozzle is also the most serious at this stage. The white coating on the surface of the heap is basically formed at this stage.
In addition to gold, the components in the gold ore heap leach liquor mainly have the following substances:
1. Humic acid salt
Due to the multi-line oxidized ore in the heap leaching ore, close to the surface, the weathered ore contains organic matter, especially the humic acid content of humic acid is high. These substances dissolve in alkaline solution to form humate, which can be Activated carbon or resin adsorption; under acidic conditions, humate will form a large molecular weight humic acid precipitate, deposited in the pores of the adsorbent, resulting in a decrease in the specific surface area of ​​the adsorbent, a decrease in adsorption capacity, and often an adsorption-desorption Equipment and pipe fittings are fouled.
Second, carbonate compounds
It is a major factor in fouling in gold mine heap leaching. It is well known that there are complex equilibriums of carbonic acid and bicarbonate compounds in aqueous solutions, generally expressed by the following reaction formula:
CO 2 +H 2 O ![]() H 2 CO 3
H 2 CO 3
H 2 CO 3 ![]() H + +HCO 3 -
H + +HCO 3 -
HCO 3 - ![]() H + +CO 3 2 -
H + +CO 3 2 -
The above three reaction formulas can be integrated into
CO 2 +H 2 O ![]() (H 2 CO 3 )
(H 2 CO 3 ) ![]() H + +HCO 3 -
H + +HCO 3 - ![]() 2H + +CO 3 2 -
2H + +CO 3 2 -
Since one CO 2 can only produce one H 2 CO 3 , one H 2 CO 3 can only ionize one HCO 3 − , and one HCO 3 − can only ionize one CO 3 2 − , so in one system, regardless of How does the concentration of CO 2 , HCO 3 - , and CO 3 2 - change, and the sum of the three concentrations is always equal to a constant C, or
![]() =1 (A)
=1 (A)
also because
 =K 1 (B)
=K 1 (B)
 =K 2 (C)
=K 2 (C)
If the three fractions in (A) are used as the dependent variable, [H + ] as the independent variable, and the three equations are solved, the CO 2 in different systems [H + ] (or pH) can be obtained. The content of HCO 3 - and CO 3 2 - . K 1 (4.45 × 10 -7 ) and K 2 (4.96 × 10 -11 ) at 25 ° C are known numbers, using three fractions as the ordinate and pH as the abscissa, which is obtained as shown in Fig. 1. The relationship between the carbonic acid compound and the pH in an aqueous solution at 25 ° C.

Figure 1 Relationship between ionization of carbonic acid and pH (25 ° C)
It can be seen from Fig. 1 that when the pH is lower than 4.4, only CO 2 is present in the solution. When the pH is > 8.35, there is no CO 2 in the aqueous solution; when the pH is 4.4 to 12.0, the bicarbonate ion HCO 3 - can exist. When the pH value is 8.35, the content of HCO 3 - is the highest, accounting for 98%; when the pH is > 8.35, there is CO 3 2 - in the aqueous solution.
The gold heap leaching solution has a pH of 9-11, so a large amount of CO 3 2 - is present in the leaching solution.
Third, calcium and magnesium ions
Calcium and magnesium ions in the heap leaching of gold from the two aspects, one, most heap leaching of gold instead of caustic lime for protection alkali solubility of lime is not large, but the closed circulation is heap leaching, long-term use, and the solution The calcium in the continually increases. Second, and most importantly, calcium and magnesium ions are mainly derived from ore. Because of the presence of organic matter in the ore, the oxidation and decomposition of the organic matter will produce CO 2 , especially in the washing stage, the CO 2 produced consumes the alkali of the leaching solution, CO 2 + OH - = HCO 3 - , and the pH of the leaching slag is lowered. And dissolves the carbonate minerals in the ore to form a large amount of bicarbonate Ca(HCO 3 ) 2 and Mg(HCO 3 ) 2 . This soluble bicarbonate combines with CO 3 2 - to form CaCO 3 or The scale of MgCO 3 .
In addition, some studies have pointed out that there is ion exchange between sodium ions and calcium and magnesium ions in the ore during leaching, that is, the calcium and magnesium ions in the ore under the sodium ion exchange in the leaching solution not only cause calcium and magnesium ions to enter the solution. Fouling is formed, and when a large amount of clay is present, the sodium ions cause the clay to swell, which also causes the original porosity of the heap to decrease and the permeability to deteriorate.
Four, silicate
SiO 2 dissolved in acid, but slightly soluble in alkali due to the constant, so there are some silicate ore leaching solution with the silicate, aluminosilicate heap leaching of gold in contact with alkaline solution, they form complex For example, a salt of metasilicate H 2 SiO 3 , ortho silicic acid H 4 SiO 4 , polysilicic acid H 2 Si 2 O 5 or the like. These silicates have low solubility, little ionic state, and mostly colloids. Further, depending on the pH, when pH=6, the amount of SiO 3 2 - is only 0.01 mg ∕ L, and when pH = 7, 0.1 mg ∕ L, pH = 10.5, the amount of dissolution is greatly increased. The amount of SiO 2 in the leachate of the US Zotman heap leaching field is 18-23 mg ∕L. The silicate compound in the leachate is very unstable, especially the colloidal silicic acid compound, which tends to be the initial nucleus of the scale. It is easy to scale on the surface of the heap as well as the carbon adsorption and resolution system.
Five, iron compounds
Alkaline leaching solution does not dissolve iron very much, but when the ore is acidic, if it contains a large amount of humic acid, the situation is different. There is more Fe 2 + in the leaching solution, because in alkaline solution, Fe 2 + is easily oxidized by oxygen in the air to form Fe 3 + , which leads to the formation of Fe(OH) 3 precipitate.
Stainless steel thick-film heating tubes of GIDAPE®ANDETONG® series manufactured by JIEDA company are tubular structures, which allows rapid water flow and instant heat carrying. Under the same conditions, GIDAPE® ANDETONG® series has many application advantages, such as large heating area, high heat conduction efficiency (≥98%), rapid thermal response (≥80 ℃~150℃/S), long working life (≥10000 hours), etc.
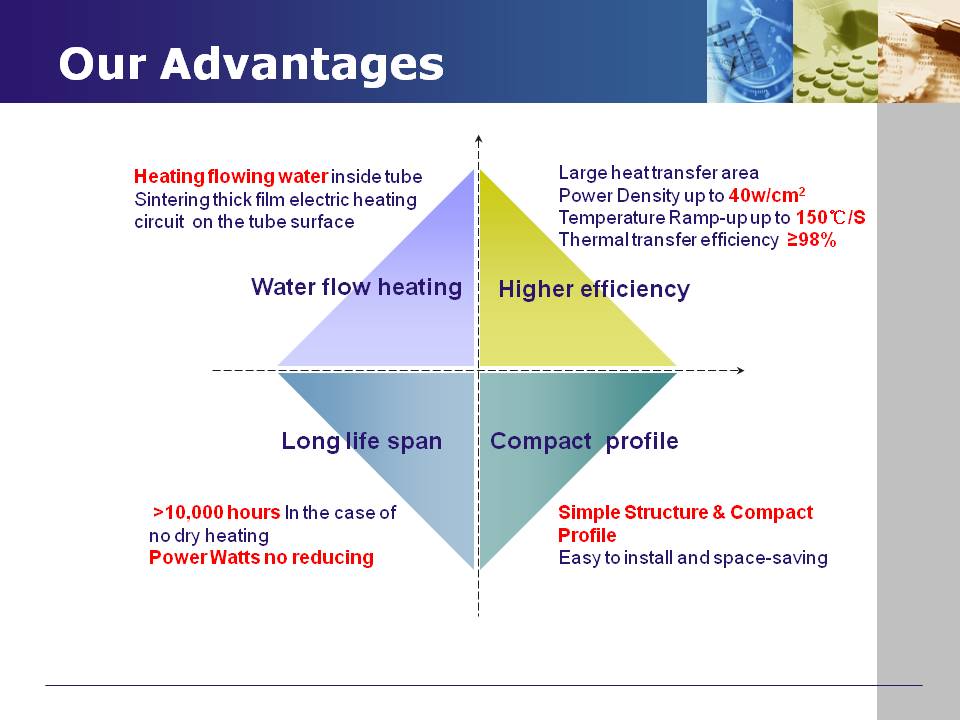
JIEDA larger than 3300w thick film Heating Element consists of stainless steel thick-film heating pipe, outer protective shell, water inlet and outlet joints, anti-dry thermostat, power cord, etc. It can be fixed on sides, with G1/2 thread diameter connecting orifice.
This type of heating body is an intelligent temperature control thick film heating body. It can work with the PCB circuit control system, so that the pre-set temperature and hot water quantity can be decided.
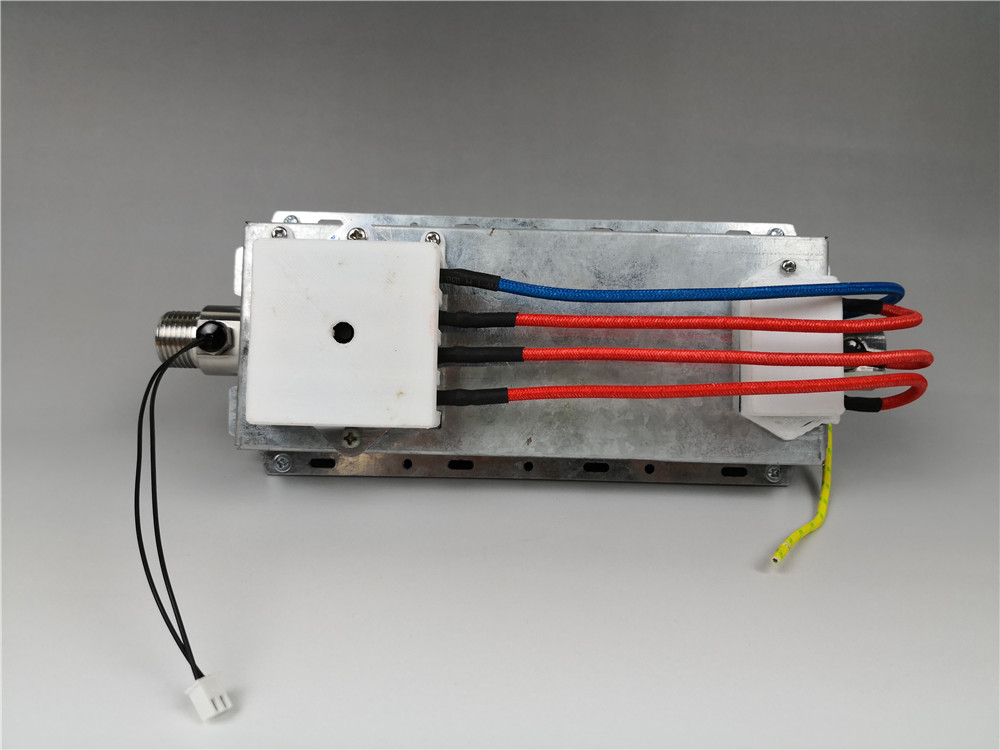
At present, thick film Heating Tube(heating body) of GIDAPE®ANDETONG® series has been successfully applied to fluid heating field, such as: pipeline machine, kitchen under sink water heater,industrial equipment and so on.
Larger Than 3300W Heating Element
Larger Than 3300W Heating Element,Water Heating Element,Thick Film Heating Element,Dishwasher Heating Elements
XINXIANG JIEDA PRECISION ELECTRONICS CO.,LTD , https://www.gidaheater.com